A study by the Medicines and Healthcare Products Regulatory Agency into reported side effects from covid vaccinations has found the majority are mild to moderate.
The report, covering the period December 9- January 24 looks at yellow cards – voluntarily reports of any suspected adverse reactions or side effects – for the Pfizer/BioNTech and Oxford/AstraZeneca vaccines.
The Pfizer/BioNTech vaccine was evaluated in clinical trials involving more than 44,000 participants. The most frequent adverse reactions in trials were pain at the injection site, fatigue, headache, myalgia (muscle pains), chills, arthralgia (joint pains), and fever.
The Oxford University/AstraZeneca vaccine was evaluated in clinical trials involving more than 23,000 participants. The most frequently reported adverse reactions in these trials were injection-site tenderness, injection-site pain, headache, fatigue, myalgia, malaise, pyrexia (fever), chills, arthralgia, and nausea;.
The majority of adverse reactions to both vaccines were mild to moderate in severity and usually resolved within a few days.
Adverse reactions reported after the second dose were milder and reported less frequently than after the first dose.
The MHPRA says these types of reactions reflect the normal immune response triggered by the body to the vaccines. They are typically seen with most types of vaccine and tend to resolve within a day or two.
The nature of Yellow Card reporting means that reported events are not always proven side effects. Some events may have happened anyway, regardless of vaccination. This is particularly the case when millions of people are vaccinated, and especially when most vaccines are being given to the most elderly people and people who have underlying illness.
The MHPRA report is based on detailed analysis of data up to January 24. At that date, an estimated 5.4 million first doses of the Pfizer/BioNTech vaccine and 1.5 million doses of the Oxford University/AstraZeneca vaccine had been administered, and around 0.5 million second doses, mostly the Pfizer/BioNTech vaccine, had been administered.
As of 24 January 2021, for the UK:
- 16,756 Yellow Cards reported for the Pfizer/BioNTech
- 6,014 reported for the Oxford University/AstraZeneca vaccine (roll out of the vaccine started later than the Pfizer one)
- 50 have been reported where the brand of the vaccine was not specified
- For both vaccines the overall reporting rate is around 3 Yellow Cards per 1,000 doses administered.
Widespread use of the vaccine suggests that severe allergic reactions to the Pfizer/BioNTech vaccine are very rare (less than 1 in 10,000 people) and have been reported at a rate between 1 and 2 cases per 100,000 doses administered.
The MHPRA says: “The overall safety experience with both vaccines is so far as expected from the clinical trials. Based on current experience, the expected benefits of both COVID-19 vaccines in preventing COVID-19 and its serious complications far outweigh any known side effects
“As with all vaccines and medicines, the safety of COVID-19 vaccines is being continuously monitored. Many suspected adverse reactions (ADRs) reported on a Yellow Card do not have any relation to the vaccine or medicine and it is often coincidental that they both occurred around the same time.
“The reports are continually reviewed to detect possible new side effects that may require regulatory action, and to differentiate these from things that would have happened regardless of the vaccine or medicine being administered, for instance due to underlying or undiagnosed illness.”
People vaccinated up to and including February 4:
First dose: 10,971,047
Second dose: 505,993
The UK seven day rolling rate of positive cases up to February 1 was 228 per 100,000 people. In Thanet the rate is below the UK average at 205 per 100,000 – a significant reduction from rates on December 20 which stood at 639 per 100,000.

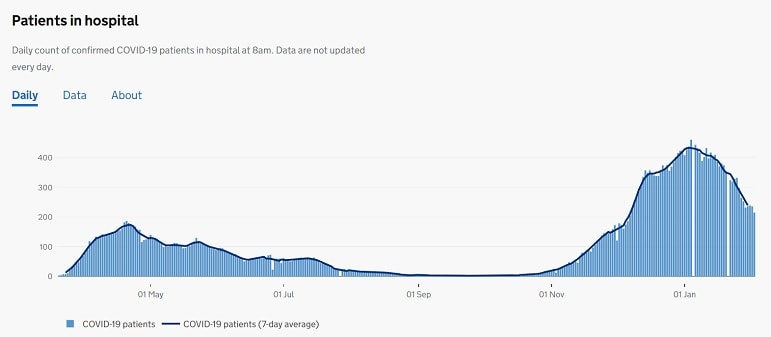
As of February 2, there were 215 patients being treated for covid in East Kent hospitals with 28 people on mechanical ventilation.
Kent and Medway vaccinations as of January 31
279,363
First dose
- over 80 years 84,547 (86.3%)
- 75-79 years 50,991 (74.3%)
- 70-74 years 27,495 (27.1%)
- under 70 years 93,726
Second dose
- over 80 years 16,083 (16.4%)
- 75-79 years 256 (0.4%)
- 70-74 years 185 (0.2%)
- under 70 years 6,080

