The government has ordered 40 million doses of a new Covid vaccine being produced by pharmaceutical firms Pfizer – which has a base in Sandwich – and /BioNTech.
The vaccine has been tested on over 40,000 volunteers and interim results suggest it is proving 90 per cent effective at protecting people against the virus. But those findings need to be peer-reviewed.

The tests were split between vaccinated individuals and those who received a placebo and results indicated a vaccine efficacy rate above 90%, at 7 days after the second dose.
This means that protection is achieved 28 days after the initiation of the vaccination, which consists of a two-dose schedule.
The Phase 3 clinical trial of the vaccine began on July 27 and has enrolled 43,538 participants to date, 38,955 of whom have received a second dose of the vaccine candidate as of November 8.
At a public briefing yesterday (November 9) Prime Minister Boris Johnson said: “If the Pfizer vaccine passes all the rigorous safety checks and is proved to be effective then we will begin a UK-wide NHS led programme of vaccine distribution.
“We will decide the order in which people are offered the vaccination taking account of recommendations from a group of scientific experts, the Joint Committee on Vaccination and Immunisation.
“They’re looking at a range of factors, including the different characteristics of different types of vaccines, to work out the most effective way to protect as many people as possible and save as many lives as we can.
“But, I must stress, these are very, very early days.”
He added: “We absolutely cannot rely on this as a solution. The biggest mistake we could make now would be to slacken our resolve at such a critical moment.”
Dr. Albert Bourla, Pfizer Chairman and CEO, said: “The first set of results from our Phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine’s ability to prevent COVID-19.
“We are reaching this critical milestone in our vaccine development program at a time when the world needs it most with infection rates setting new records, hospitals nearing over-capacity and economies struggling to reopen.
“We are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis. We look forward to sharing additional efficacy and safety data generated from thousands of participants in the coming weeks.”
“The first interim analysis of our global Phase 3 study provides evidence that a vaccine may effectively prevent COVID-19. This is a victory for innovation, science and a global collaborative effort,” said Prof. Ugur Sahin, BioNTech co-founder and CEO.
“When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality. We will continue to collect further data as the trial continues to enroll for a final analysis planned when a total of 164 confirmed COVID-19 cases have accrued.
The Pfizer/BioNtech vaccine is one of 11 currently in the final stages of testing.
The companies now plan to apply for emergency approval to use the vaccine by the end of November – and a limited number of people may get the vaccine this year.
Military and NHS staff are on standby to roll out a Covid-19 vaccine across the UK.
Deputy chief medical officer Professor Jonathan Van-Tam said he was “hopeful” there would be “some vaccine by Christmas”.
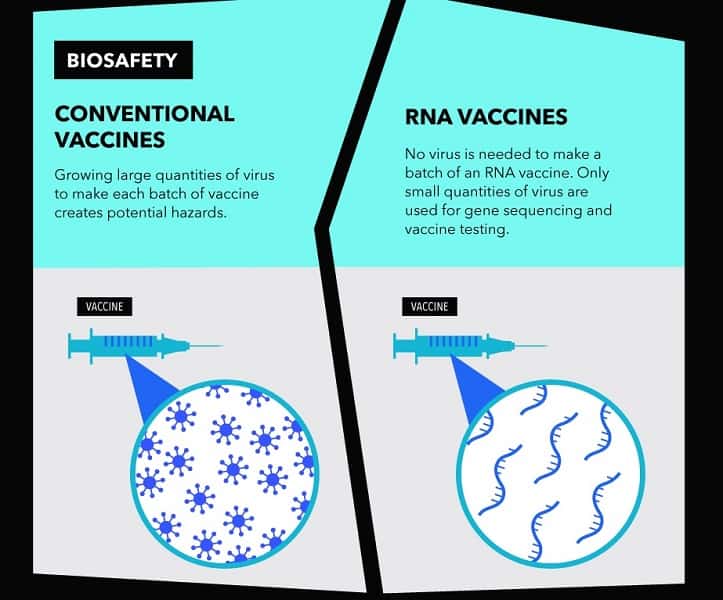
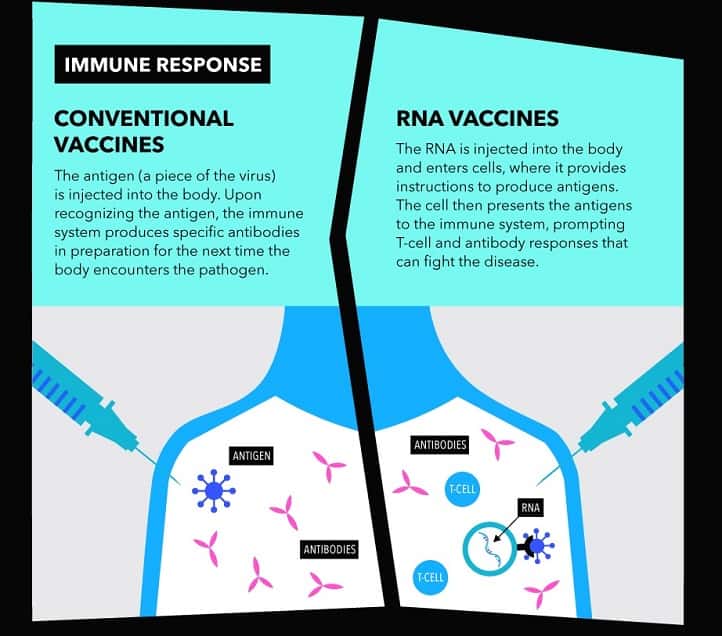
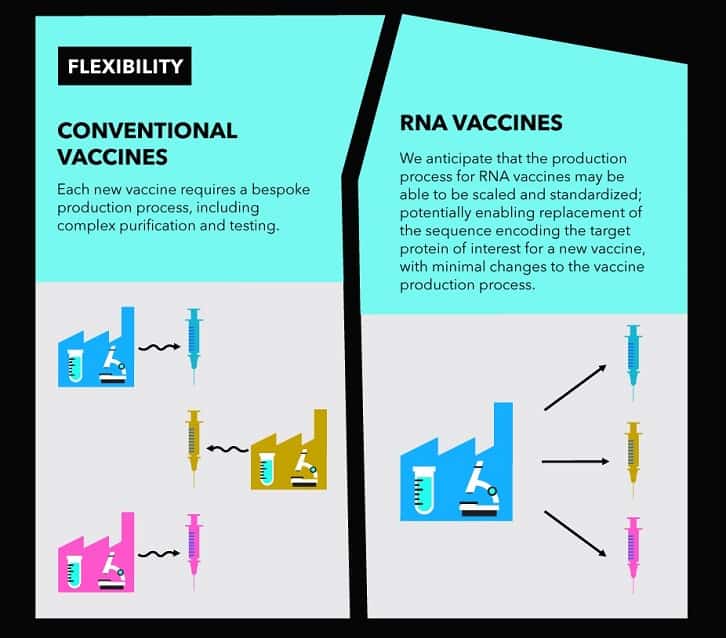
It is a new type of vaccine called an mRNA vaccine and uses a fragment of the virus’ genetic code. This starts making part of the virus inside the body, which the immune system recognises as foreign and starts to attack. There are no mRNA vaccines that have been approved for use in people.

